Oxyacids of Phosphorus: Preparation, Structure, Properties and Uses
Oxyacids of Phosphorus: Preparation, Structure, Properties and Uses: Overview
This topic covers concepts, such as, Oxoacids of Phosphorus, Structures of Oxoacids of Phosphorus, Preparation of Oxoacids of Phosphorus & Properties of Oxoacids of Phosphorus etc.
Important Questions on Oxyacids of Phosphorus: Preparation, Structure, Properties and Uses
The number of bonds in the structure of phosphorous pentaoxide and phosphorous trioxide are respectively.()
The structure of is
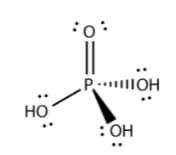
Write the balanced reaction for the preparation of the following acid. Pyrophosphoric acid
How many linkage (s) are present in ?
For next two question please follow the same
Phosphorus forms a number of oxoacids which differ in their structures and oxidation state of phosphorus. All the acids contain phosphorus atom/atoms linked tetrahedrally to four other atoms or groups. Each of them has at least one P = O or unit and one P - OH unit. The -OH group is ionisable but H atom linked directly to P is non-ionisable. Structures of all the acids are considered to be derived either from phosphorus acid or phosphoric acid.
Which one is mono-basic acid?
Which among the following oxoacids of phosphorus shows a tendency of disproportionation?
The structure of is as follows
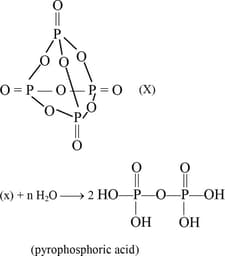
What is the value of ?
What is the oxidation state of phosphorous in , and ?
Name and draw the structures of different oxyacids of phosphorous.
What are the basicity of and
Why does behave as reducing agent?
Give the disproportionate reaction of .
What happens when is heated?
What is the basicity of ?
When white phosphorous reacts with alkali, then the produced oxoacid is?
From the given acids the phosphinic acid is?
The product obtained by hydrolysis of among the following is:
Among the following, which is the reducing acid?
Among the following, the most stable anion is:
The pair of acids in which phosphorous atom have formal oxidation state of +3 is
